
Arvind Sahu, Ph.D.
arvindsahu@nccs.res.in
Education
- Ph.D., 1991, Vallabhbhai Patel Chest Institute, University of Delhi, Delhi.
- M.Sc., 1985, Dept. of Biochem. & Microbiol., Nagpur University, Nagpur
- B.Sc., 1983, J. B. College of Science (Wardha), Nagpur University, Nagpur
Professional Experience
- Currently Executive Director, Regional Centre for Biotechnology, Faridabad.
- Scientist G, 2012-2023, National Centre for Cell Science, Pune.
- Scientist F, 2007-2012, National Centre for Cell Science, Pune.
- Scientist E, 2004-2007, National Centre for Cell Science, Pune.
- Scientist D, 2000-2004, National Centre for Cell Science, Pune.
- Research Assistant Professor, 1998-2000, University of Pennsylvania, Philadelphia, Pennsylvania
- Post-doctoral training, 1994-1998, University of Pennsylvania, Philadelphia, Pennsylvania. 1991-1994, University of Texas Health Sci. Ctr. at Tyler, Texas.
Research
Viruses are among the most successful pathogens that co-existed with the hosts by maintaining a precarious balance between the two for their successful existence. Being predatory in nature, viruses are constantly in the pursuit of survival, and thus, there exists a constant struggle for endurance between the viruses and their hosts: viruses pursue the host for their propagation, and the hosts, on the other hand, defy the viral intrusions owing to their well-developed and interconnected network of innate and adaptive immune defense mechanisms. Our laboratory focuses on these assiduous struggles between viruses and the immune system, in particular between viruses and the complement system.
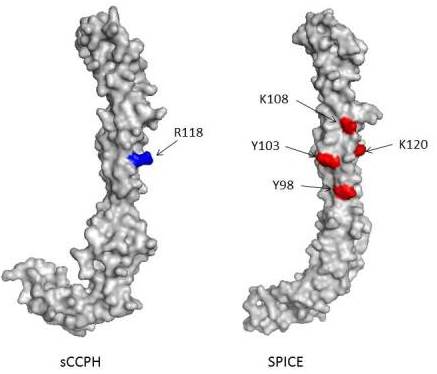
Model of sCCPH (HVS complement regulator) and SPICE (variola virus complement regulator) showing 'hot spots' important for complement regulation.
Viral complement evasion
The complement system is an ancient mechanism of immunological defense that evolved to perform surveillance and protect the host from all the pathogenic non-self targets, including viruses. Although viruses are small and have relatively simple structures, both acute and latent viruses can be efficiently recognized and neutralized by the complement system. Thus, to combat host responses and succeed as pathogens, along with other immune evasion mechanisms, viruses must also develop principles to elude the host complement system. Consistent with this premise, genome sequencing of pox and herpesviruses has shown that these viruses encode for homologs of the human complement regulators. Our laboratory has shown that the homolog encoded by Kaposi’s sarcoma-associated herpesvirus (KSHV) is functional, and we named it Kaposica. In addition, we and others have also shown that complement regulators encoded by KSHV and other viruses (vaccinia virus, variola virus, and Herpesvirus saimiri) inhibit complement by targeting the C3-convertases. Our group has also contributed to mapping the functional domains, determinants, and species specificity of these viral proteins. Our studies revealed that the ‘functional blocks’ required for complement regulatory activities in the viral regulators are conserved in the human regulators as well, and this led to the construction of a dual-activity human DAF-MCP chimera. Currently, studies in our laboratory are directed towards understanding the role of virally encoded complement regulators in viral pathogenesis.
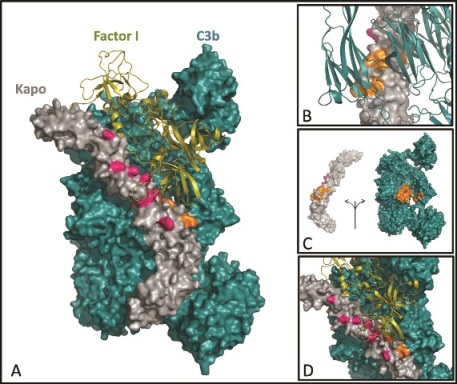
Mapping of the functional sites in C3b-Kaposica (Kapo)-factor I trimolecular complex. (A) Model of C3b-Kapo-factor I trimolecular complex. C3b (cyan) and Kapo (gray) are represented by solid surface, whereas factor I is represented by cartoon (olive). Residues of Kapo that affect its activity are labeled in orange and pink. (B) Zoomed view of C3b interaction sites (orange) of Kapo. C3b domains are represented by cartoon (cyan). (C) Footprint of Kapo interaction sites on C3b. The footprints are seen in MG2 and CUB domains in C3b. (D) Zoomed view of factor I contact sites (pink) of Kapo.
Role of complement during viral infections
Initially, for many decades, it was believed that the complement system targets pathogens owing to its direct effect on them. But we now know that complement also boosts the acquired immune system. Specifically, it was shown that coupling of the complement fragment to antigen lowers the threshold of B cell activation as well as prolongs antigen retention by the FDCs. In addition, complement also participates in priming of both CD4+ and CD8+ T cells by stimulation of the complement receptor-mediated signalling in T cells as well as affecting the antigen-presenting cell function. Intriguingly, recent findings have shown that complement activation occurs even intracellularly, signifying likely cross-talks of the complement system with many other cellular pathways. Thus, work in our laboratory focuses on understanding the role of intact complement as well as individual pathways during viral infections. Our lab showed that synergy between the classical and alternative pathways is critical for providing effective protection against the pandemic influenza A(H1N1)2009 virus infection. Further, our data indicated that complement synthesized by the splenic B cells and C3a receptor-mediated signalling plays a major role in providing the protection. Currently, our lab is using both in vivo and in vitro approaches to further dissect the immunological basis of protection provided by the complement system against viral infections.
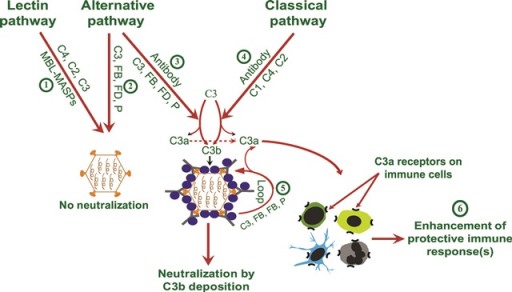
Model for the interplay between classical and alternative pathways during A(H1N1)pdm09 virus infection.
Publications
Selected Publications Publications Patents
Selected Publications
All Publications
|
Shende, R., Wong, S.S.W., Meitei, H.T., Lal, G., Madan, T., Aimanianda, V., Pal J.K., Sahu, A. (2022) Protective role of host complement system in Aspergillus fumigatus infection. Front. Immunol. 13:978152. PMID: 31036650 DOI: 10.3389/fimmu.2022.978152 |
| Mastellos, D.C., Ricklin, D., Sfyroera, G., Sahu, A. (2022) From discovery to approval: A brief history of the compstatin family of complement C3 inhibitors. Clin. Immunol., 235: 1-5. PMID: 34147650 DOI: 10.1016/j.clim.2021.108785 |
| Ghate, A.*, Sharma, S.*, Agrawal, P. and Sahu, A. (2021) Differential expression of complement receptors CR1/2 and CR4 by murine M1 and M2 macrophages. Mol. Immunol., 137: 75-83. PMID: 34229135 DOI: 10.1016/j.molimm.2021.06.003 |
| Sinha, A., Singh, A.K., Kadni, T.S., Mullick, J., Sahu, A. (2021) Virus-Encoded Complement Regulators: Current Status. Viruses, 13, 208. PMID: 33573085 DOI: 10.3390/v13020208 |
| Nawadkar, R., Dravid, A., Paprunia, U., Lewis, R., Kojima, K., Abraham, P., and Sahu, A. (2021) Case report of laboratory-acquired vaccinia virus infection in India. Weekly Epidemiological Record – WHO, 96 (5/6): 33-44. |
| Narkhede, Y.B., Gautam, A.K., Hsu, R.V., Rodriguez, W., Zewde, N.T., Harrison, R.E., Arantes, P.R., Gaieb, Z., Gorham Jr, R.D., Kieslich, C., Morikis, D., Sahu, A., Palermo, G. (2021) Role of electrostatic hotspots in the selectivity of complement control proteins toward human and bovine complement inhibition. Front. Mol. Biosci. 8:618068. PMID: 33829039 DOI: 10.3389/fmolb.2021.618068 |
| Agrawal, P., Sharma, S., Pal, P., Ojha, H., Mullick, J. and Sahu, A. (2020) The imitation game: a viral strategy to subvert the complement system. FEBS Letters, 594: 2518–2542. PMID: 32506518 DOI: 10.1002/1873-3468.13856 |
| Maitra, A., Raghav, S., Dalal, A., Ali, F., Paynter, V.M., Paul, D., Biswas, N.K., Ghosh, A., Jani, K., Chinnaswamy, S., Pati, S., Sahu, A., Mitra, D., Bhat, M.K., Mayor, S., Sarin, A., The PAN-INDIA 1000 SARS-CoV-2 RNA Genome Sequencing Consortium, Shouche, Y.S., Seshasayee, A.S.N., Palakodeti, D., Bashyam, M.D., Parida, A., and Das, S. (2020) PAN-INDIA 1000 SARS-CoV-2 RNA Genome Sequencing Reveals Important Insights into the Outbreak. bioRxiv 2020.08.03.233718. DOI: 10.1101/2020.08.03.233718 |
| Paul, D., Jani, K., Kumar, J., Chauhan, R., Seshadri, V., Lal, G. Karyakarte, R., Joshi, S. Tambe, M., Sen, S., Karade, S., Anand, K.B., Shergill, S.P.S., Gupta, R.M., Bhat, M.K., Sahu, A., Shouche, Y.S. (2020) Phylogenomic analysis of SARS-CoV-2 genomes from western India reveals unique linked mutations. bioRxiv 2020.07.30.228460. DOI: 10.1101/2020.07.30.228460 |
Panwar, H.S., Ojha, H., Ghosh, P., Barage, S.H., Raut, S. and Sahu, A. (2019) Molecular engineering of an efficient four-domain DAF-MCP chimera reveals the presence of functional modularity in RCA proteins, Proc. Natl. Acad. Sci. USA., 116(20): 9953-9958. PMID: 31036650 DOI: 10.1073/pnas.1818573116 |
| Ojha, H., Ghosh, P., Panwar, H.S., Shende, R., Gondane, A., Mande, S.C. and Sahu, A. (2019) Spatially conserved motifs in complement control protein domains determine functionality in regulators of complement activation-family proteins, Commun. Biol., 2:290: 1-12. DOI: 10.1038/s42003-019-0529-9 |
Kumar, A., Phulera, S., Rizvi, A., Sonawane, P.J., Panwar, H.S. Banerjee, S., Sahu, A. and Mande, S.C. (2019) Structural basis of hypoxic gene regulation by the Rv0081 transcription factor of Mycobacterium tuberculosis, FEBS Letters, 593(9): 982-995. PMID: 30941756 DOI: 10.1002/1873-3468.13375 |
Puraswani, M., Khandelwal, P., Saini, H., Saini, S., Gurjar, B.S., Sinha, A., Shende, R.P. et al. (2019) Clinical and immunological profile of anti-factor H antibody associated atypical hemolytic uremic syndrome: A nationwide database, Front. Immunol., 10:1282: 1-12. PMID: 31231391 DOI: 10.3389/fimmu.2019.01282 |
Shende, R., Wong, S.S.W., Rapole, S., Beau, R., Ibrahim-Granet, O., Monod, M., Gührs, K-H, Pal, J.K., Latgé, J-P., Madan, T., Aimanianda, V. and Sahu, A. (2018) Aspergillus fumigatus conidial metalloprotease Mep1p cleaves host complement proteins, J. Biol. Chem., 293(40): 15538–15555. PMID: 30139746 DOI: 10.1074/jbc.RA117.001476 |
Gurjar, B., Sriharsha, T., Bhasym, A., Prabhu, S., Puraswani, M., Khandelwal, P., Saini, H., Saini, S., Verma, A., Chatterjee, P., Guchhait, P., Bal, V., George, A., Rath, S., Sahu, A., Sharma, A., Hari, P., Sinha, A., Bagga, A. (2018) Characterization of genetic predisposition and autoantibody profile in atypical hemolytic uremic syndrome, Immunology, 154: 663–672. PMID: 29485195 DOI: 10.1111/imm.12916 |
Wong, S.S.W., Rani, M., Dodagatta-Marri, E., Ibrahim-Granet, O., Kishore, U., Bayry, J., Latgé, J-P, Sahu, A., Madan, T. and Aimanianda, V. (2018) Fungal melanin stimulates surfactant protein D-mediated opsonization of and host immune response to Aspergillus fumigatus spores, J. Biol. Chem., 293(13): 4901-4912. PMID: 29414772 DOI: 10.1074/jbc.M117.815852 |
Rattan, A., Pawar, S.D., Nawadkar, R., Kulkarni, N., Lal, G., Mullick, J., and Sahu, A. (2017) Synergy between the classical and alternative pathways of complement is essential for conferring effective protection against the pandemic influenza A(H1N1) 2009 virus infection, PLoS Pathog, 13(3): 1-26. PMID: 28301559 DOI: 10.1371/journal.ppat.1006 |
| Agrawal,P., Nawadkar, R., Ojha, H., Kumar, J. and Sahu, A. (2017) Complement evasion strategies of viruses: an overview, Front. Microbiol., 8: 1-19. PMID: 28670306 DOI: 10.3389/fmicb.2017.01117 |
Kumar, J., Yadav, V.N., Phulera, S., Kamble, A., Gautam, A.K., Panwar, H.S. and Sahu, A. (2017) Species specificity of vaccinia virus complement control protein towards bovine classical pathway is governed primarily by direct interaction of its acidic residues with factor I., J. Virol., 91(19): e00668-17. PMID: 28724763 DOI: 10.1128/JVI.00668-17 |
| Khuperkar, D., Kamble, A., Singh, A., Ghate, A., Nawadkar, R. Sahu, A. and Joseph, J. (2017) Selective recruitment of nucleoporins on vaccinia virus factories and the role of Nup358 in viral infection, Virology, 512: 151–160. PMID: 28963881 DOI: 10.1016/j.virol.2017.09.0 |
Gautam, A.K., Panse, Y., Reza, J.M, Mullick, J. Ghosh,P., and Sahu, A. (2015) Mutational analysis of Kaposica reveals that bridging of MG2 and CUB domains of target protein is crucial for the cofactor activity of RCA proteins, Proc Natl Acad Sci USA, 112(41): 12794-9. PMID: 26420870 DOI: 10.1073/pnas.1506449112 |
| Ojha, H., Panwar, H.S., Gorham Jr., R.D., Morikis, D. and Sahu, A. (2014) Viral regulators of complement activation: structure, function and evolution, Mol Immunol, 61: 89-99. PMID: 24976595 DOI: 10.1016/j.molimm.2014.06 |
| Reza, M.J, Kamble, A., Ahmad, M., Krishnasastry, M.V. and Sahu, A. (2013) Dissection of functional sites in Herpesvirus saimiri complement control protein homolog, J Virol, 87(1): 282-295. PMID: 23077301 DOI: 10.1128/JVI.01867-12 |
| Rattan, A., Kasbe, R., Mullick, J. and Sahu, A. (2013) The complement system as a viral target for immune evasion, Chapter 1 In Microbial Pathogenesis: Infection and Immunity U Kishore and A Nayak, editors Landes Bi, pp: 1-27. |
Yadav, V. N., Pyaram, K., Ahmad, M. and Sahu, A. (2012) Species selectivity in poxviral complement regulators is dictated by the charge reversal in the central complement control protein modules, J Immunol, 189(3): 1431-1439. PMID: 22732591 DOI: 10.4049/jimmunol.1200946 |
| Dinasarapu, A.R., Chandrasekhar, A., Sahu, A., and Subramaniam, S. (2012) Complement C3, UCSD-Nature Molecule Pages, : -. DOI: 106072/H0MPA00423501 |
Bernet, J., Ahmad, M., Mullick, J., Panse, Y., Singh, A.K., Parab, P.B. and Sahu, A. (2011) Disabling complement regulatory activities of vaccinia virus complement control protein reduces vaccinia virus pathogenicity, Vaccine, 29(43): 7435-43. PMID: 21803094 DOI: 10.1016/j.vaccine.2011.07 |
Pyaram, K., Kieslich, C.A., Yadav, V.N., Morikis, D. and Sahu, A. (2010) Influence of electrostatics on the complement regulatory functions of Kaposica, the complement inhibitor of Kaposi’s sarcoma-associated herpesvirus, J Immunol, 184(4): 1956-1967. PMID: 20089702 DOI: 10.4049/jimmunol.0903261 |
| Kadam, A.P. and Sahu, A. (2010) Identification of Complin, a novel complement inhibitor that targets complement proteins factor B and C2, J Immunol, 184(12): 7116-7124. PMID: 20483772 DOI: 10.4049/jimmunol.1000200 |
Ahmad, M., Raut, S., Pyaram, K., Kamble, A., Mullick, J. and Sahu, A. (2010) Domain swapping reveals complement control protein modules critical for imparting cofactor and decay-accelerating activities in vaccinia virus complement control protein, J Immunol, 185(10): 6128-6137. PMID: 20956343 DOI: 10.4049/jimmunol.1001617 |
| Pyaram, K., Yadav, V.N., Reza, M.J. and Sahu, A. (2010) Virus-complement interactions: an assiduous struggle for dominance, Future Virol, 5(6): 709-730. |
Singh, A. K., Yadav, V.N., Pyaram, K., Mullick, J. and Sahu, A. (2009) Mapping of functional domains in Herpesvirus saimiri complement control protein homolog: the complement control protein domain 2 is the smallest structural unit displaying cofactor and decay-accelerating activities, J Virol, 83(19): 10299-10304. PMID: 19640995 DOI: 10.1128/JVI.00217-09 |
Yadav, V. N., Pyaram, K., Mullick, J. and Sahu, A. (2008) Identification of hot spots in the variola virus complement inhibitor (SPICE) for human complement regulation, J Virol, 82(7): 3283-3294. PMID: 18216095 DOI: 10.1128/JVI.01935-07 |
Ahmad, M., Pyaram, K., Mullick, J. and Sahu, A. (2007) Viral complement regulators: the expert mimicking swindlers, Ind J Biochem Biophy, 44: 331-343. PMID: 18341208 |
Soulika, A.M., Holland, M. C. H., Sfyroera, G., Sahu, A., and Lambris J.D. (2006) Compstatin inhibits complement activation by binding to the b-chain of complement factor 3, Mol Immunol, 43(12): 2023-2029. PMID: 16472861 |
Singh, A.K., Mullick, J., Bernet, J. and Sahu, A. (2006) Functional characterization of the complement control protein homolog of Herpesvirus saimiri: Arg-118 is critical for factor I cofactor activities, J Biol Chem, 281: 23119-23128. PMID: 16760474 |
Mullick, J., Singh, A.K., Panse, Y., Yadav, V., Bernet, J. and Sahu, A. (2005) Identification of functional domains in kaposica, the complement control protein homolog of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8), J Virol, 79(9): 5850-5856. PMID: 15827200 |
Mullick, J.*, Bernet, J.*, Panse, Y., Hallihosur, S., Singh, A.K. and Sahu, A. (2005) Identification of complement regulatory domains in vaccinia virus complement control protein, J Virol, 79(19): 12382-12393. PMID: 16160165 |
Bernet, J., Mullick, J., Panse, Y., Parab, P.B. and Sahu, A. (2004) Kinetic analysis of the interactions between vaccinia virus complement control protein and human complement proteins C3b and C4b, J Virol, 78(17): 9446-9457. PMID: 15308738 |
| Sarrias, M.R., Sahu, A., and Lambris, J.D. (2004) Characterization of the interactions of complement receptor 2 with its ligands iC3b, C3d and EBV glycoprotein gp350/220, Biacore Journal, 4(1): 16-19. |
| Sahu, A., Morikis, D., and Lambris, J.D. (2003) Compstatin, a peptide inhibitor of complement, exhibit species-specific binding to complement component C3 , Mol Immunol, 39: 557-566. PMID: 12431389 |
Mullick, J., Bernet, J., Singh, A. K., Lambris, J. D. and Sahu, A. (2003) Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins, J Virol , 77(6): 3878-3881. PMID: 12610165 |
| Soulika, A.M., Morikis, D., Sarrias, M.R., Roy, M., Spruce, L.A., Sahu, A. and Lambris J.D. (2003) Studies of structure-activity relations of complement inhibitor compstatin, J Immunol, 171(4): 1881-1890. PMID: 12902490 |
| Bernet, J., Mullick, J., Singh, A.K. and Sahu, A. (2003) Viral mimicry of the complement system, J Biosci, 28(3): 249-264. PMID: 12734404 |
| Mullick, J., Kadam, A. and Sahu, A. (2003) Herpes and pox viral complement control proteins: ‘the mask of self’, Trends Immunol, 24(9): 500-507. PMID: 12967674 |
| Morikis, D., Roy, M., Sahu, A., Troganis, A., Jennings, P.A., Tsokos, G.C., Lambris, J.D. (2002) The structural basis of compstatin activity examined by structure-function-based design of peptide analog and NMR, J Biol Chem, 277(17): 14942-53. |
| Sahu, A. and Lambris, J.D. (2002) Invited Commentary on Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass, Ann, Thorac Surg, 74(2): 362-. |
Franchini, S., Zarkadis, I.K., Sfyroera, G., Sahu, A., Moore, W.T., Mastellos, D., Lapatra, S.E. and Lambris, J.D. (2001) Cloning and purification of the rainbow trout fifth component of complement (C5), Dev Comp Immunol, 25(5-6): 419-430. PMID: 11356221 |
Sarrias, M.R., Franchini, S., Canziani, G., Moore, W.T., Sahu, A., Lambris, J.D. (2001) Kinetic analysis of the interactions of complement receptor 2 (CR2, CD21) with its ligands C3d, iC3b and the Epstein Barr virus glycoprotein gp350/220, J Immunol, 167(3): 1490-1499. PMID: 11466369 |
| Sahu, A. and Lambris, J.D. (2001) Structure and biology of complement protein C3, a connecting link between innate and acquired immunity, Immunol Rev, 180: 35-48. PMID: 11414361 |
| Saha, K. and Sahu, A. (2001) Has Multi-Drug Therapy (MDT) Any Adverse Effects On Female Reproduction?, The Star , 60(1): 12-15. |
| Sahu, A., Soulika, A.M., Morikis, D., Spruce, L., Moore, W.T. and Lambris, J.D. (2000) Binding kinetics, structure-activity relationship and biotransformation of the complement inhibitor Compstatin, J Immunol, 165: 2491-2499. PMID: 10946275 |
Soulika, A.M., Khan, M., Hattori, T., Bowmen, F.W., Richardson, B.A., Sahu, A., Edmunds Jr, H.L. and Lambris, J.D. (2000) Inhibition of heparin/protamine complex-induced complement activation by Compstatin in baboons, Clin Immunol, 95: 212-221. PMID: 10964539 |
| Sahu, A., Morikis, D., and Lambris, J.D. (2000) Complement inhibitors targeting C3, C4, and C5, Chapter 4 In Therapeutic Interventions in the Complement System J D Lambris and V M Holers, editors, pp: 75-112. |
| Sahu, A. and Lambris, J.D. (2000) Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics, Immunopharmacology, 49(1-2): 133-148. PMID: 10904113 |
Sun, X., Funk, C.D., Deng, C., Sahu, A., Lambris, J.D., and Song, W. C (1999) Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting, Proc Natl Acad Sci USA, 96: 628-633. PMID: 9892684 |
| Sahu, A., Rawal, N. and Pangburn, M.K. (1999) Inhibition of complement by covalent attachment of rosmarinic acid to activated C3b, Biochem Pharmacol, 57: 1439-1446. PMID: 10353266 |
Fiane, A.E., Mollnes, T.E., Videm, V., Hovig, T., Høgåsen, K., Mellbye, O.J., Spruce L., Moore, W.T., Sahu, A., Lambris, J.D. (1999) Prolongation of ex vivo-perfused pig xenograft survival by the complement inhibitor Compstatin Transplant, Transplant Proc, 31: 934-935. PMID: 10083413 |
Fiane, A.E., Mollnes, T.E., Videm, V. Hovig, T., Høgåsen, K., Mellbye, O.J., Spruce, L. Moore, W.T. Sahu, A., and Lambris, J.D. (1999) Compstatin, a peptide inhibitor of C3, prolongs survival of ex vivo perfused pig xenografts, Xenotransplantation, 6: 52-65. PMID: 10355733 |
| Lubinski, J., Wang, L., Mestellos, D., Sahu, A., Lambris, J.D., Friedman, H.M. (1999) In vivo role of complement interacting domains of herpes simplex virus type 1 glycoprotein gC, J Exp Med, 190(11): 1637-1646. PMID: 10587354 |
| Morikis, D., Sahu, A., Moore, W.T., and Lambris, J.D. (1999) Design, structure, function and applications of Compstatin, In Bioactive Peptides in Drug Discovery and Design: Medical Aspects J Matsoukas and T Mavromoustako, pp: 235-246. |
| Morikis, D., Assa-Munt, N. and Sahu, A., Lambris, J.D. (1998) Solution structure of Compstatin, a potent complement inhibitor, Protein Science, 7(3): 619-627. PMID: 9541394 |
Sahu, A., Isaacs, S.N., Soulika, A.M. and Lambris, J.D. (1998) Interaction of vaccinia virus complement control protein with human complement proteins: Factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway, J Immunol, 160: 5596-5604. PMID: 9605165 |
Nilsson, B., Hong, J., Larsson, R., Elgue, G., Nilsson-Ekdahl, K., Sahu, A., Lambris, J. D. (1998) Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation, Blood , 92(5): 1661-1667. PMID: 9716594 |
| Lambris, J. D., Sahu A., and Wetsel R. (1998) The chemistry and biology of C3, C4, and C5, Chapter 5 in The Human Complement System in Health and Disease J E Volanakis and M Frank, editors , pp: 83-118. |
Sahu, A., Sunyer, J.O., Moore, W.T., Sarrias, M.R., Soulika, A.M., Lambris, J.D. (1998) Structure, functions, and evolution of the third complement component and viral molecular mimicry, Immunologic Research , 17(1&2): 109-121. PMID: 9479573 |
Kostavasili, I*., Sahu, A*., Friedman, H.M., Eisenberg, R.J., Cohen, G.H. and Lambris, J.D. (1997) Mechanism of complement inactivation by glycoprotein C of herpes simplex virus, J Immunol, 158: 1763-1771. PMID: 9029114 |
| Sahu, A. and Pangburn, M.K. (1996) Investigation of mechanism-based inhibitors of complement activation targeting the thioester site of C3, Biochem Pharmacol, 51: 797-804. PMID: 8602875 |
Sunyer, J.O., Zarkadis, I.K., Sahu, A. and Lambris, J.D. (1996) Multiple forms of complement C3 in trout that differ in binding to complement activators, Proc Natl Acad Sci USA, 93: 8546-8551. PMID: 8710907 |
| Sahu, A., Kay, B.K. and Lambris, J.D. (1996) Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library, J Immunol, 157: 884-891. PMID: 8752942 |
| Sahu, A. and Pangburn, M.K. (1995) Tyrosine is a potential site for covalent attachment of activated complement component C3, Mol Immunol, 32(10): 711-716. PMID: 7659097 |
Sahu, A., Kozel, T.R. and Pangburn, M.K. (1994) Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation, Biochem J, 302(2): 429-436. PMID: 8092994 |
Sahu, A. and Pangburn, M.K. (1994) Covalent attachment of human complement C3 to IgG: Identification of the amino acid residue involved in ester linkage formation, J Biol Chem, 269(46): 28997-29002. PMID: 7961863 |
| Dash, K., Saha, K., Sahu, A. and Gangal, S.V. (1993) Natural serum haemagglutinins (lectins) in fish: Physicochemical characterization, Fish & Shellfish Immunol, 3: 345-360. |
Saha, K., Dash, K. and Sahu, A. (1993) Antibody dependent haemolysin, complement and opsonin in sera of a major carp, Cirrhina mrigala and catfish, Clarius batrachus and Heteropneustes fossilis, Comp Immunol Microbiol & Infec Dis, 16(4): 323-330. PMID: 8281746 |
| Sahu, A. and Pangburn, M.K. (1993) Identification of multiple sites of interaction between heparin and the complement system, Mol Immunol, 30(7): 679-684. PMID: 8487783 |
| Kashyap, A., Sehgal, V.N., Sahu, A. and Saha, K. (1992) Anti-leprosy drugs inhibit the complement-mediated solubilization of pre-formed immune complexes in-vitro, Int J Immunopharmac, 14(2): 269-273. PMID: 1624226 |
| Sahu, A., Saha, K., Mukherjee, A. and Sehgal, V.N. (1992) In vivo effects of anti-leprosy drugs on the rat peritoneal macrophages and lymphocyte subpopulations, Int J Immunopharmac, 14(4): 721-730. PMID: 1325957 |
Kashyap, A., Saha, K. Sahu, A., Chakrabarty, A.K. and Chattopadhyay, D. (1992) Delayed clearance of circulating immune complexes in mice following administration of anti-leprosy drugs, Int J Leprosy, 60(3): 404-409. PMID: 1474278 |
Sahu, A., Saha, K., Banerjee, N., Sehgal, V.N. and Jagga, C.R. (1991) Effect of anti-leprosy drugs on superoxide anion production by rat peritoneal macrophages with special reference to light exposed clofazimine, Int J Immunopharmac, 13(4): 419-428. PMID: 1646774 |
| Saha, K. and Sahu, A. (1990) Transfer factor as a probe of the immune defect in lepromatous leprosy (HD), The Star , 49(7): 8-10. |
| Sahu, A., Saha, K., Kashyap, A. and Chakrabarty, A.K. (1988) Interaction of anti-leprosy drugs with the rat serum complement system, Immunopharmacology, 5(3): 143-150. PMID: 1624226 |
| Rambukkana, A., Saha, K., Sahu, A. and Chopra, K. (1988) Undernutrition and altered T-cell homeostasis in children with severe chest diseases, J Trop Pediat, 34: 282-288. PMID: 3146649 |
| Saha, K., Sahu, A. and Chakrabarty, A.K. (1986) Immunological aspects of lepra reaction, In Proceedings of Workshop on Reaction in Leprosy Indian Association of Leprologists, New Delhi, pp: 109-115. |
Patents
|
1. Lambris, J.D. and Sahu, A. (2001) Peptides which inhibit complement activation. USA patent #6,319,897 |
|
2. Sahu, A. and Kadam, A.P. Peptides that inhibit factor B, C2 and complement activation, and their uses. (Application #889/DEL/2010 dated 13.4.2010). Indian Patent #2884803. Sahu, A., Ojha, H., Ghosh, P., Barage and Panwar, H.S. (2019) DAF-MCP chimeric protein, process to manufacture the same and use of the chimeric protein for treating pathological conditions involving the complement system. US Application #: PCT/IN2020/050337 filed on April 9, 2020. |
|
3. Sahu, A., Ojha, H., Ghosh, P., Barage and Panwar, H.S. (2019) DAF-MCP chimeric protein, process to manufacture the same and use of the chimeric protein for treating pathological conditions involving the complement system. US Application #: PCT/IN2020/050337 filed on April 9, 2020. |
Awards and Achievements
Wellcome Trust Overseas Senior Fellow in Biomedical Science in India (2001-2006)
Fellowship/Membership of Scientific Society
- Fellow, Indian Academy of Sciences, Bengaluru, India (2019)

- Fellow, Indian National Science Academy, New Delhi, India (2016)

- Fellow, National Academy of Sciences, India (2009)

- Member, International Complement Society (1993 - present)

- Member, American Association of Immunologists (1997-2001)
- Member, Molecular Immunology Forum (2005-present)
- Member, American Society for Microbiology (2005-present)
Lab Members
Renuka Nawadkar (Ph.D. student)
Palak Agrawal (Ph.D. student)
Pradipta Pal (Ph.D. Student)
Samriddhi Sharma (Ph.D. student)
Anup Kumar Singh (Ph.D. student)
Subhasis Sahu (Ph.D. student)
Deoraj Mogre (Technical Officer A)
Lab Alumni
Ph.D. students and Post-Doctoral fellows
Jayati Mullick, Ph.D.
(Current Affiliation: Scientist E & Group Leader, Avian Influenza Division, National Institute of Virology, Pune)
John Bernet, Ph.D.
(Current Affiliation: Scientist, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram)
Akhilesh K. Singh, Ph.D.
(Current Affiliation: Post-doc at Seattle Children's Research Institute, University of Washington, Seattle, WA, USA).
Archana Kadam, Ph.D.
(Taken a career break to bring up her family).
Muzammil Ahmad, Ph.D.
(Current Affiliation: Scientist, Elixirgen, LLC, USA)
Kalyani Pyaram, Ph.D.
(Current Affiliation: Post-doc at University of Michigan Medical School, Ann Arbor, MI, USA)
Vivekanand Yadav, Ph.D.
(Current Affiliation: Post-doc at University of Michigan Medical School, Ann Arbor, MI, USA)
Malik Reza, Ph.D.
(Current Affiliation: Post-doc at Children's Hospital Oakland Research Institute, Oakland, CA, USA)
Avneesh Kumar Gautam, Ph.D.
(Current Affiliation: Post-doc at Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA)
Ajitanuj Rattan, Ph.D.
(Current Affiliation: Post-doc at University of Rochester Medical School, Rochester, NY, USA)
Ashish Kamble, Ph.D.
(Current Affiliation: Senior Research Officer, Vaccine Research and Development Centre (VRDC), Shikrapur, Pune)
Jitendra Kumar, Ph.D.
(Current Affiliation: Post-doc at Baylor Institute for Immunology Research, Dallas Texas, USA)
Hemendra Singh Panwar, Ph.D.
(Current Affiliation: Post-doc at UT Southwestern Medical Center, Dallas, Texas, USA.)
Hina Ojha, Ph.D.
(Current Affiliation: Post Doctoral Fellow at University of Dundee, Scotland, UK)
Rajashri Shende, Ph.D.
(Current Affiliation: Associate Research Analyst, Intox Private Limited, Pune)
Arya M. Ghate, Ph.D.
Back
Last updated On : 04 March 2025 11:17


